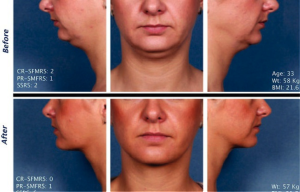
Have you ever wished your double chin would just go away? Then you may be excited to learn about a new injectable that was just approved by the FDA. Kybella™ is just like the deoxycholic acid made by your own human body, which absorbs fat. However, when injected precisely into the submental fat, it zaps fat cells, but it is only designed and approved for the chin fat at this time.
It is very important to note that while this is exciting news for people who want to get rid of their double chin, there is extreme caution necessary because Kybella™ can inadvertently destroy other types of cells besides its target of fat cells, if not properly injected. You must go through a highly trained, board certified plastic surgeon to avoid having the wrong types of cells eliminated, such as skin cells, for example.
Patients who heed this warning may be clamoring to give the new Kybella™ a try. It is expected to revolutionize double chin problems in patients who feel conscious about that “turkey neck” syndrome but who do not want to go through a full-blown facelift procedure.
What to Expect with Kybella™
The Med Spa at Southwest Plastic Surgery of El Paso, TX and Las Cruces, NM will be one of the first in the United States to offer Kybella™ for patients who want to get rid of their double chin. If you are interested in getting the procedure, here are some things you may want to know…
- Patients can get up to fifty Kybella™ injections within one single treatment, with as many as six exclusive treatments performed about one month apart for each.
- Kybella™ should never be mixed or diluted with any other chemicals/compounds.
- This new double-chin fat blaster was deemed safe based upon two, separate clinical trials comprised of over one thousand adults; all of whom had moderate-to-serious submental chin fat. Patients chosen were given either Kybella™ or placebo treatments randomly of up to six sessions, but those participants who experienced reduction in chin fat were the patients who were given Kybella™.
- The use of Kybella™ outside of the intended area is not approved and is not recommended. People who have any type of injections in the jaw or mouth area should not be given Kybella™.
- Some side effects may occur, such as redness, numbness, swelling or bruising in the treatment area, although these are not common. Although rare, patients should be aware of potentially serious effects, such as nerve injury, trouble swallowing or muscle weakness in the face. That is why it is so critical for patients to use only a board certified, licensed physician. They should also be aware that Kybella™ comes in a package with a distinctive hologram; hence if the hologram is not present, the product should never be used.
Overall, the potential for Kybella™ as an ideal solution for double chins is extremely promising and exciting to people who have been self-conscious about this part of their face. After its recent FDA approval, Kybella™ should be available at quality plastic surgery centers in the very near future, as early as this summer of 2015. Contact Southwest Plastic Surgery to find out when Kybella™ will be available!